Classes I IIa IIb and III. Validity and Renewal.

Pharmaboardroom Regulatory Pricing And Reimbursement Malaysia
Malaysias Medical Device Authority Has Published the Fifth Edition of the Requirements for Medical Device Labeling August 2022.

. Vision Mission Core Value. The Government of Malaysia is fairly reasonable allowing us to get personal tax reliefs from lifestyle expenses such as gadgets and sports equipment to mandatory ones such as education and medical expenses for our parents and ourselves. Once issued an MA License does not expire.
In sections 32 and 422 as the directives 9342EEC on medical devices and 90385EEC on active implantable medical devices have been fully repealed on 26 May 2021 by Regulation EU no. Currently medical devices do not require. For the full list of personal tax reliefs in Malaysia as of the.
The purpose of the device typically falls under one. NATIONAL INSTITUTES OF HEALTH NIH. Medical device registration is handled by Turkish Medicines and Medical Devices Agency TITCK CLASSIFICATION.
NATIONAL BLOOD CENTRE. MEDICAL DEVICE AUTHORITY MALAYSIA. Medical Device Directive 2015 3 3 Subject to the provisions of this Agreement each.
Medical device manufacturers must appoint and grant Power-of-Attorney to a local registered company in Vietnam to submit registration applications and act as the Registration Holder. The MDR Technical Documentation Template must be submitted to Notified Body or Competent Authority for review and approval. NATIONAL CANCER INSTITUTE.
These Guidance Document was prepared by the Medical Device Authority MDA to help the industry and healthcare professionals in their quest to comply with. We would like to show you a description here but the site wont allow us. Needs further updates esp.
Besides medical cleansers EtO is used in a range of products including antifreeze textiles plastics detergents and adhesives. This article needs to be updatedThe reason given is. Official Portal of Medical Device Authority MDA Malaysia.
A guide on the SOP to enter Malaysia has been prepared and applies to the following international students as. Our Hotline 603 - 8230 0300. Theres even a tax relief for alimony payments.
5th September 2022. We would like to show you a description here but the site wont allow us. It should be preferably made in the English language or in an official language of an.
For medium to high-volume settings our ABL800 FLEX blood gas analyzer offers a high throughput and reliable automated sample handling with Drop n Go capability which means theres no need to wait at the analyzer for results. Radiometer offers you a wide selection of blood gas analyzers with features and functionalities that match your facilitys needs. We are Australias government authority responsible for evaluating assessing and monitoring products that are defined as therapeutic goods.
Clifford Chance has advised Asian Development Bank European Bank for Reconstruction and Development First Abu Dhabi Bank International Finance Corporation and Natixis in the transaction. General country-specific regulatory information is provided on this page for medical device registration and approval in Qatar. Not applicable LOCAL FEES.
Any party who wishes to know whether an establishment who is. We would like to show you a description here but the site wont allow us. Medical devices in Qatar are managed by the Ministry of Economy and Commerce MEC.
Medical Device Regulations and Classification in Qatar. Furthermore Brexit triggers updates in these sections. Democratic Republic Malaysia the Republic of the Union of Myanmar the Republic of the Philippines the Republic of.
With your permission we and our partners would like to use cookies in order to access and record information and process personal data such as unique identifiers and standard information sent by a device to ensure our website performs as expected to develop and improve our products and for advertising and insight purposes. All Import Licenses expire on December 31 2022. The section related to EU.
We protect the health of Australians by regulating medicines and medical devices for safety quality and effectiveness. This facility is provided to enable any interested parties to search for Registered Medical Device Registered CAB Licensed Establishments under Section 5 10 15 of Medical Device Act 2012 Act 737 respectively and Notified Medical Device under Medical Device Exemption Order 2016. Qualtech is an international medical device consulting firm operating in Asia North America and EU.
With a focus on Regulation Market Access and Clinical Trials. The latest Standard Operating Procedure SOP on international students entering Malaysia is available on the Education Malaysia Global Services webpage. The Government of Malaysia has agreed to allow the entry of international students effective from 1 st January 2021.
It also used to decontaminate some food products and spices. Product Notification in Product Tracking System UTS will take approximately 1 week. Medical devices encompass a broad range of instruments equipment apparatus in-vitro diagnostic reagents calibrators software and consumables intended for the purpose of detecting measuring restoring correcting or modifying the structure or function of the body for a medical purpose.
2 ACCESS PAGE Calendar. Representative shall register the medical device with the Regulatory Authority of that Member State. Medical Device Authority MDA Ministry of Health Malaysia Level 6 Prima 9 Prima Avenue II Block 3547 Persiaran APEC 63000 Cyberjaya Selangor MALAYSIA 603 - 8230 0300 603 - 8230 0200.
MEDICAL DEVICE AUTHORITY MDA MINISTRY OF HEALTH MALAYSIA. MDR 2017 745 Annex II Medical Device Technical File is a summary document prepared by the manufacturer in a clear well-organized. We regulate medicines medical devices and biologicals to help Australians stay.
February 27 2020 Genuine Diamond Sdn Bhd
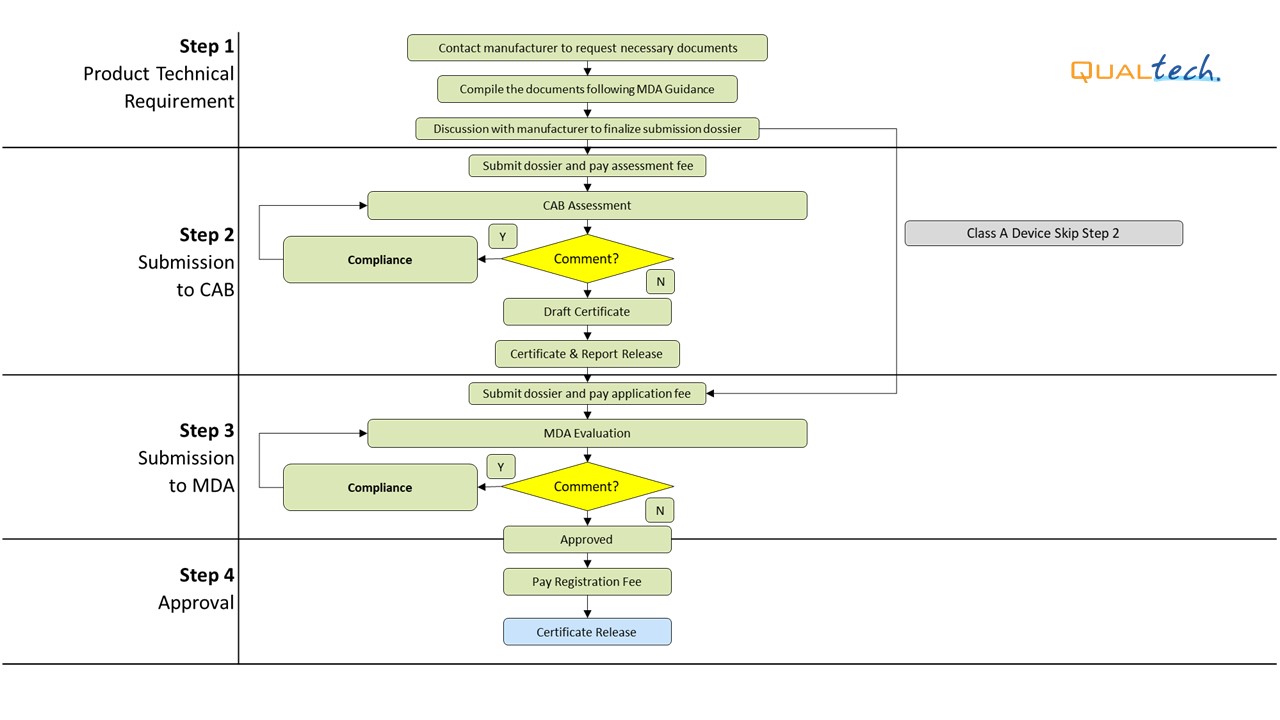
General Medical Device Medical Device Authority Mda

Central Medicare Central Medicare
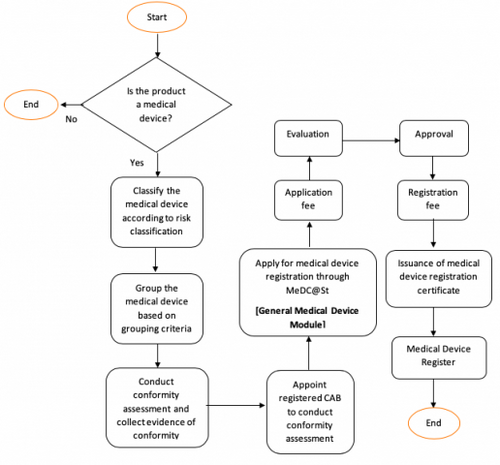
General Medical Device Medical Device Authority Mda

Mihir Shah On Twitter News Flash Ibreastexam Is Now Approved For Commercial Sales In Malaysia Just Received Product Approval From The Medical Device Authority Usfda Ce Mark Cofepris India Nepal Botswana Kenya

Specobag 什么是 Mda Certificate Mda Medical Device Facebook

Mda On Complaint Handling And Problem Reporting

Medical Device Authority Mda Facebook
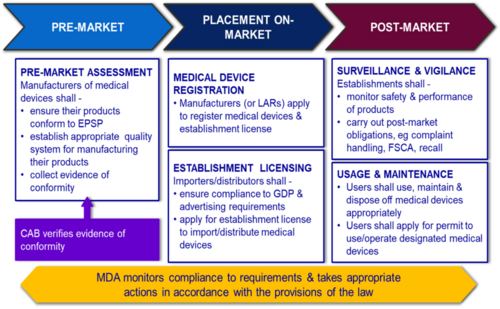
General Medical Device Medical Device Authority Mda
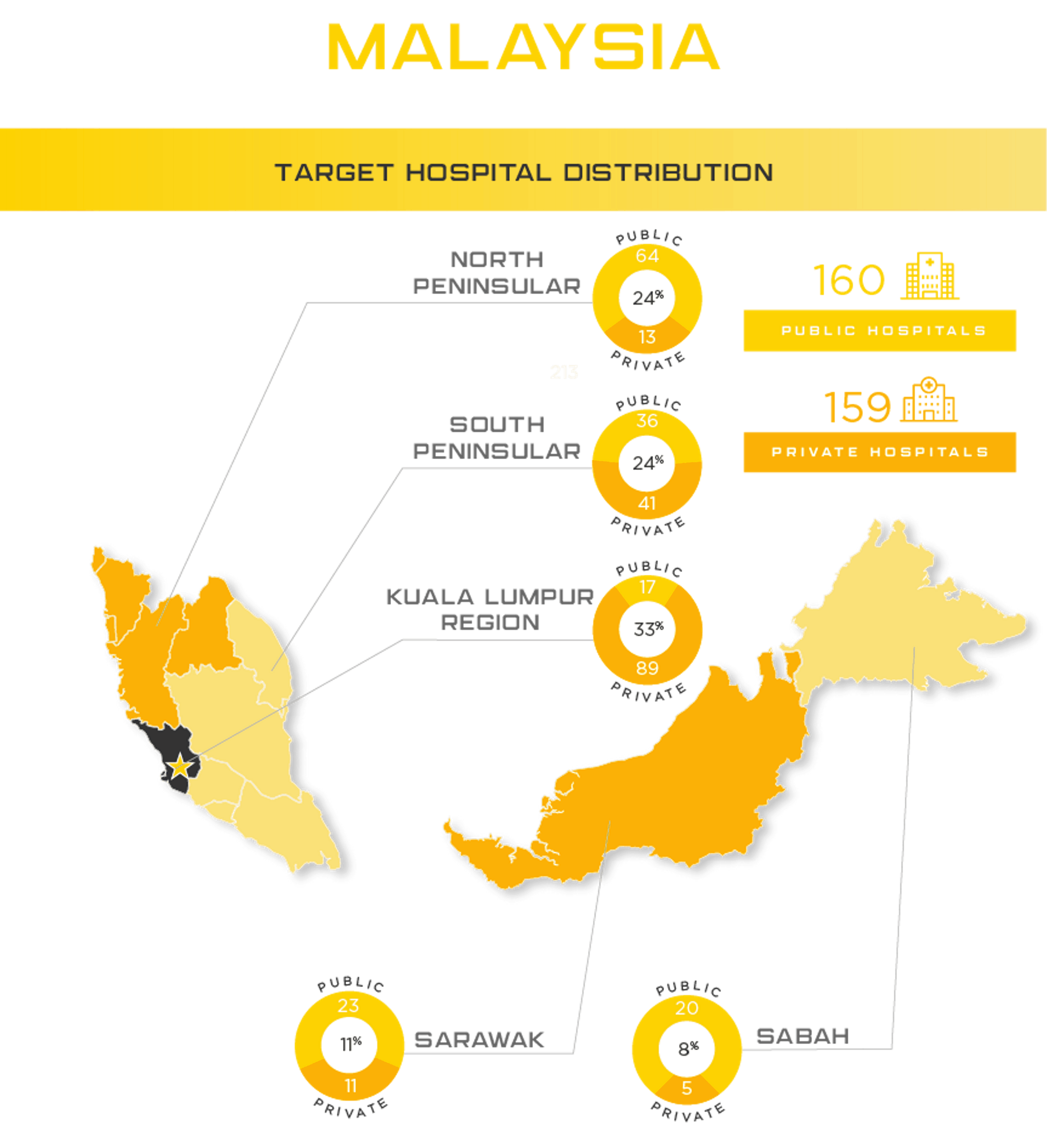
Malaysia Medical Device Registration License Holding Asia Actual
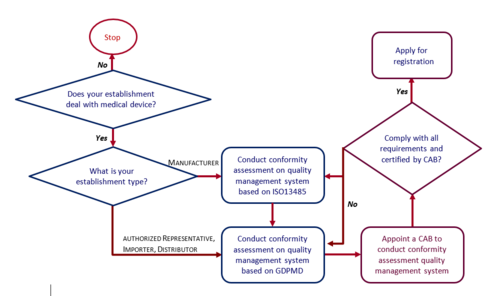
How To Apply For Establishment Licence Medical Device Authority Mda

Medical Device Registration In Malaysia

Public Search Malaysia Medical Device Register Mmdr

Medical Device Authority Mda Facebook
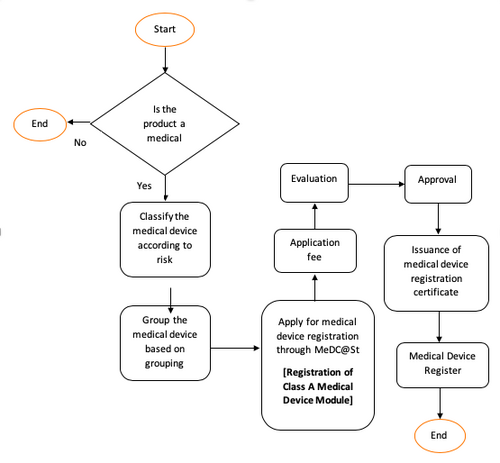
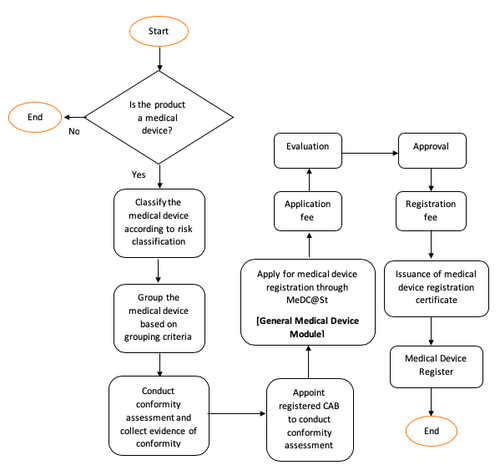
General Medical Device Medical Device Authority Mda